Is the success value of a covid vaccine important.
Several vaccines against Covid 19 have been introduced in various countries and institutions around the world. There is also the question of what is the best vaccine. Because while the effectiveness of some vaccines is around 95%, some countries buy vaccines that have a success rate of 63%. For example, the Pfizer / BioNTech vaccine has a 95% success rate while the Oxford / AstraZeneca imported to Sri Lanka has a success rate of 63.09%. So when there are more successful vaccines, why do we get less successful vaccines? Why are they producing more? If only the best one was distributed to the world, it would be over! These questions inevitably come to our minds.
But in reality the success of a vaccine cannot be compared to that percentage. This article explains why.
How to measure the success of a vaccine
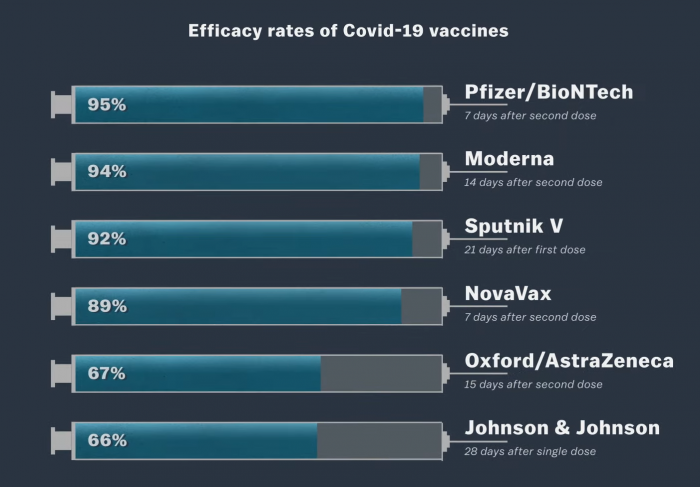
The following is a diagram showing the success rates of vaccines currently being distributed in the world.
Let’s explain this story in terms of how a vaccine can measure success. The success of a vaccine is measured by the large number of clinical trials. Research by tens of thousands of people. Half of the random human samples selected in this way were vaccinated for testing. The same vaccine is given for the other half, but it has no medicinal value and no harm to the body. But they are not informed. In short, both those who received the correct vaccine and those who received the fake vaccine do not know whether they were given the correct one or not. Such a counterfeit drug is called placebo in medicine. Doctors prescribe drugs for patients who do not have a physical illness but claim to have it. “The doctor sees and the disease is cured,” says Ann.
Somehow half were vaccinated and the other half were given placebo and the two were given the opportunity to live a normal life for a few months. Meanwhile, scientists are waiting to see if they can catch the virus. More than 43,000 such samples have been used to test the Pfizer / Bio Entech vaccine. Of these, 21,500 have been vaccinated with Pfizer / Bio Entech and the other 21,500 with placebo. By the end of the research period, 170 of them had been infected with the corona virus.
Now the important thing is which category the 170 belongs to. If the vaccine is divided equally between the injected group 85 and the remaining placebo groups 85, the vaccine efficiency is 0%. So, why is it the same whether the vaccine is given or not? If all 170 are in placebo, the vaccine is 100% effective if it does not cause the disease. The value of the placebo kit in the Pfizer / Bio Entech vaccine is 162. The remaining 8 were in the vaccinated group. Then the success rate of that vaccine is 95%.
Now, just because the vaccine is 95% effective does not mean that 5 out of 100 people will get corona. That 95% applies to one person who gets the vaccine. This means that a person who has been vaccinated has a 95% lower risk of developing coronary heart disease every time they are exposed to the corona virus. That’s what a vaccine is.
Each vaccine is successful in the same way as above. Why can the success of vaccines not be compared with those values? There are several reasons for this. The key is the timing and area of clinical trials for each of these vaccines.
Can the success rate between vaccines be compared?
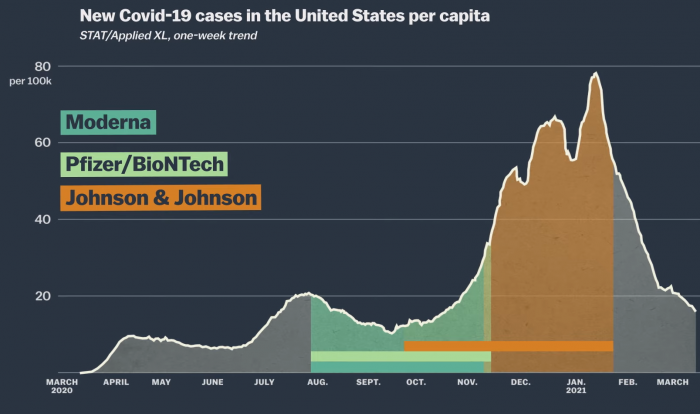
All of the Moderna vaccine research was done only in the United States. Pfizer was also primarily researched in the United States. Both vaccines were researched between August and November 2020. It was during that time that the second wave of corona in the United States ruled. However, the research period for the Johnson & Johnson vaccine was between October 2020 and January 2021. It was during this time that the third corona wave began and a large number of patients were reported. It is clear that those who received the J&J vaccine were more likely to be exposed to the disease. This means that more people who get the vaccine are more likely to get the disease (J&J has a 66% success rate).
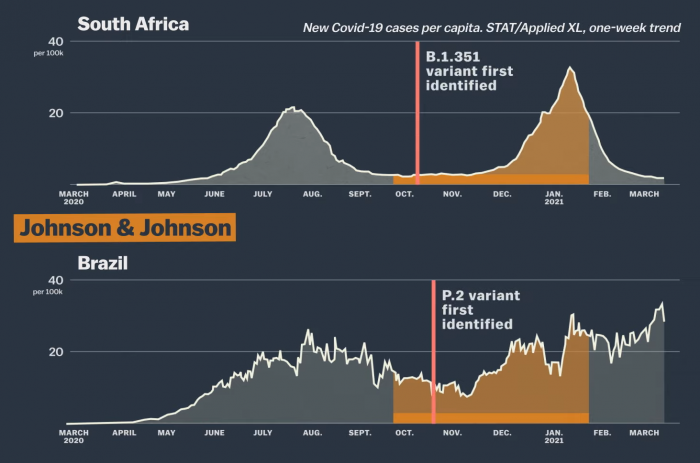
The Johnson & Johnson vaccine has been tested primarily in South Africa and Brazil. In those countries too, from October 2020 to January 2021 the number of patients had increased significantly. In addition, different strains of the corona virus were identified during their research period. The B.1.351 variant found in South Africa and the P.2 variant found in Brazil are corona variants not found in the world when the J&J vaccine was developed. So in such a situation it is practically possible for a vaccine to show an overall success rate of 66%.
Therefore, we can only compare the success of each vaccine if all the researchers did it under the same conditions. The other is that these success rates are only the result of clinical trials. In the real world, lumbering elephants are exposed by the aggression of speeding midgets. Therefore, the success value of a vaccine is not very practical (not unimportant).
Most importantly, the role of the vaccine is not always to completely prevent infection. None of these vaccines have been developed with the goal of eradicating corona from the world. The purpose of all these vaccines is to control the severity of coronary heart disease, reduce hospitalization and, most importantly, prevent death. All of these vaccines have achieved that goal. No one who received the vaccine died of coronary heart disease. This means that the effectiveness of each of these vaccines against coronary hospitalization or death is 100% effective.
The question we need to ask is not what vaccine will stop the corona infection completely, but what vaccine will keep us out of the hospital or help end the corona epidemic one day. The answer to all three questions is – “All these vaccines.”